Increased TGF-β expression in the bone marrow microenvironment is present in most cases of myeloproliferative neoplasms (MPNs), however, its contribution to disease pathogenesis is not well understood. Multiple prior studies show that TGF-β suppresses the proliferation of hematopoietic stem/progenitor cells (HSPCs) in vitro. However, its impact on HSCs in vivo is less clear, with conflicting data showing that TGF-β suppresses or induces HSPC proliferation, depending upon the dose of TGF-β and cell context. Here, we explore the hypothesis that HSPCs carrying a Jak2V617F mutation are resistant to the suppressive effects of TGF-β, conferring a fitness advantage that contributes to their expansion in MPNs.
To test this hypothesis, we first assessed TGF-β signaling in wildtype (WT) and Jak2V617F HSPCs. Whereas TGF-β1 induced robust Smad2/3 phosphorylation in WT phenotypic hematopoietic stem cells (HSCs), it was barely detectable in Jak2V617F HSCs. Interestingly, this phenotype was lost in lineage-restricted progenitors, where TGF-β1-induced Smad2/3 phosphorylation was comparable in WT and Jak2V617F cells. These data suggest that TGF-β signaling, at least Smad2/3 phosphorylation, is selectively loss in Jak2V617F HSCs but not lineage-restricted progenitors.
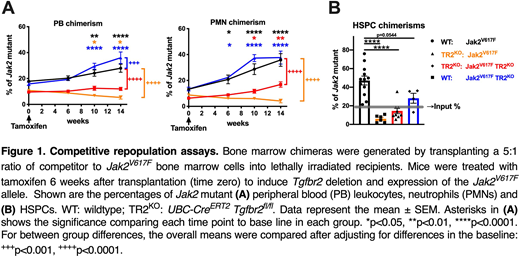
Competitive repopulation assays (CRAs) were performed using WT and Jak2V617F HSPCs in which Tgfbr2 was deleted to abrogate TGF-β signaling in HSCs. We first established bone marrow chimeras containing equal amounts of WT and UBC-CreERT2; Tgfbr2f/f HSPCs (hereafter referred to as TR2KO cells). Tamoxifen was given 6 weeks after transplantation to activate Cre expression and delete Tgfbr2. Multilineage donor chimerism of the Tgfbr2-deleted cells increased over time, suggesting that TGF-β signaling negatively regulates HSPC repopulating activity. However, when competing Jak2V617F cells with Jak2V617F TR2KO cells, the TR2KO compartment no longer had competitive advantage, suggesting that Jak2 mutated HSPC are insensitive to the suppressive effect of TGF-β. To extend these findings, CRAs were established that included the following 4 cohorts: 1) WT vs. Jak2V617F; 2) TR2KO vs. Jak2V617F; 3) TR2KO vs. Jak2V617FTR2KO; and 4) WT vs. Jak2V617FTR2KO. In each case, a 5:1 ratio of competitor to Jak2-mutated cells was used and tamoxifen was given 6 weeks after transplantation to induce both Cre-mediated deletion of Tgfbr2 and activation of the Jak2V617F allele. In cohort 1, as expected, donor chimerism with Jak2 mutated cells increased over time (Figure 1A, black). Consistent with this finding, Jak2 mutant chimerism in HSPCs was increased at 12-14 weeks (Figure 1B). In cohort 2 (orange), the loss of TGF-β signaling in WT HSPCs completely abrogated the competitive advantage of Jak2V617F HSPCs. In fact, the TR2KO cells outcompeted Jak2V617F HSPCs. In cohort 3 (red), we asked whether loss of TGF-β signaling in both WT and Jak2V617F HSPCs "leveled the playing field". Indeed, no expansion of Jak2 mutant peripheral blood leukocytes or HSPCs was observed in these mice. Finally, in cohort 4 (blue), we observed that loss of TGF-β signaling in Jak2 mutant cells had little impact on their repopulating activity.
To assess the impact of TGF-β signaling on WT and Jak2V617F HSPC proliferation, we generated bone marrow chimeras containing either WT vs. Jak2V617F HSPCs or TR2KO vs. Jak2V617FTR2KO HSPCs. Of note, in both types of bone marrow chimeras, the presence of Jak2 mutant cells is expected to increase the local level of endogenous TGF-β. As expected, in the WT vs. Jak2V617F chimeras, the Jak2V617F HSPCs were less quiescent than WT cells. Surprisingly, loss of TGF-β signaling resulted in an increase in HSC quiescence in both TR2KO and Jak2V617F TR2KO HSCs. Of note, the loss of TGF-β signaling again "leveled the playing field" with a similar percentage of quiescent TR2KO vs. Jak2V617FTR2KO HSCs.
Collectively, these data suggest that attenuated TGF-β signaling in Jak2V617F HSPCs plays a key role in the pathogenesis of MPN. Specifically, the data support a model in which increased local production of TGF-β by Jak2V617F hematopoietic cells selectively inhibits WT HSPCs, thereby promoting the expansion of Jak2V617F HSPCs. Targeting TGF-β signaling early in the course of MPN or in persons with Jak2V617F clonal hematopoiesis may prevent or delay clonal progression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal